Understanding the Basics of Chemical-Resistant Polyesters and Vinyl Esters
Gary R. Hall Consultant
Gary R. Hall, now retired, was manager of research and development at Sauereisen, Inc. (Pittsburgh, PA). He was with Sauereisen for 45 years. Hall is active in NACE, ASTM, and the American Institute of Chemical Engineers. He is a contributing editor for JPCL and also is a recipient of one of JPCL’s 2012 Top Thinker awards.
 Outside view of Bunker C crude oil storage tank with a floating roof. Surface preparation of the tank interior will be followed by application of a vinyl ester coating.
Outside view of Bunker C crude oil storage tank with a floating roof. Surface preparation of the tank interior will be followed by application of a vinyl ester coating.
All photos courtesy of Sauereisen Unsaturated polyester resins based on maleic and fumaric acids have been known since the 1920s. In the late 1930s, the modern form of these resins was introduced when Charleton Ellis combined styrene with unsaturated polyester.
Polyester and vinyl ester resins have been used in severe chemical environments for 50 years in the form of mortars for chemical-resistant brickwork and in fiberglass reinforced plastic (FRP). These successes led to the development of chemical-resistant coatings and linings based upon these resins that offer superior resistance to a broad range of chemicals, especially acids, and to higher temperatures than most other coating types like epoxies and polyurethanes.1
The terms “coatings” and “linings” are used throughout this article. For the purposes of this article, linings are composed of glass fabric, mat, or woven roving saturated with the chosen resin. They are applied in sheets of fiberglass reinforcement saturated in resin, which are then laid against the substrate and rolled in place using a ribbed roller. Coatings are usually thinner than linings and are applied as a mixture by brush, roller, airless spray, and plural component spray.
This article describes the basic chemistry of polyesters and vinyl esters, properties, concerns with the materials, and application methods.
Basic Chemistry
Unsaturated polyesters are formed by the reaction of a dibasic organic acid, such as phthalic or maleic acid, and an alcohol or polyol such as ethylene glycol. Unsaturated polyester resin (usually called a “polyester resin” or “polyester”) is a thermoset that can be cured from a liquid state under the proper conditions. A wide range of polyesters, including partially aromatic and aromatic versions, can be made by using different acids, glycols, alcohols, and monomers, each with different properties (Fig. 1).
Polyester resins used in coatings are typically pale-colored, viscous liquids consisting of the polyester dissolved in a monomer, usually styrene. Styrene reduces the viscosity of the resin, making it easier to handle. Styrene is called a reactive diluent because it is involved in the curing of the polyester resin, as well as in reducing viscosity. They cure through a free radical mechanism. The free radicals are produced by reaction of an organic peroxide, such as methyl ethyl ketone peroxide (MEKP), and a reducing agent, typically a cobalt salt. This type of free radical initiation is known as a redox (reduction-oxidation) reaction. When added to the resin, the MEKP splits into two free radicals [RO• + ROO•], each of which then react with the styrene, causing it to form another free radical.
These styrene radicals then react with the carbon-carbon double bonds (-C=C-) adjacent to the ester groups along the length of the polyester resin molecules forming cross-links between adjacent polymer molecules, without creating by-products. The uncured polyester molecule has multiple reactive sites along the length of the molecule. Multiple cross-link sites ensure that the molecules are tightly bonded to each other. This allows for high mechanical strength and excellent chemical resistance, but it also introduces rigidity to the cross-linked network. This irreversible reaction results in a dense and complex network of intertwined polymers with excellent chemical resistance. Polyester resins and vinyl ester resins are highly reactive, have a short shelf life, and will gel or set on their own upon standing. Warm temperatures hasten this reaction. Inhibitors are often added during manufacture to prolong storage life. Refrigeration is also often recommended. Even with the use of inhibitors and refrigeration, the shelf life of polyester and vinyl ester resins is typically three months or less.
Saturated dicarbonic acids are used in polyesters to control cross-link density and to optimize the properties of the cured polymer network. The three most commonly used dicarbonic acids and their contributions to the cured network are shown in the box on p. 36.
To formulate vinyl ester and polyester coatings, the coating manufacturer will add other materials, including initiator; accelerator; and typical coating raw materials such as thixotropes, fillers, and pigments. Flake glass and silane-treated micaceous iron oxide (MIO) are often preferred fillers because they beneficially reduce coating permeability. Fillers are often in the range of 45–50% by weight. There is also some evidence that flake glass will help limit the length of cracks that may form in the coating due to stresses in service.
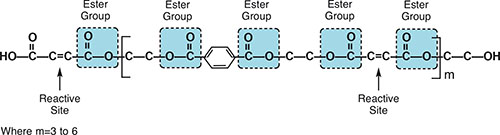 Fig. 1: Unsaturated polyester polymer.
Fig. 1: Unsaturated polyester polymer. Vinyl ester resins, a special subclass of polyesters, are similar to polyester resins in that both contain multiple ester groups and are cured in the same way. There are, however, some significant differences in the polyester resins and vinyl ester resins used to formulate corrosion-resistant coatings. The main difference is that a polyester resin molecule has several reactive ester sites along its length, while a vinyl ester resin has only two ester groups, both in the terminal or vinyl position. This has a significant effect upon the properties of the resulting cured polymer. Since the reactive sites in a vinyl ester resin are only found in the terminal positions of the chain, cross-linking can only occur at these sites, in contrast to several cross-link sites on a polyester chain. Terminal attachment between two polymer chains means that, unlike what occurs in polyester polymers, the entire polymer chain between reactive ester sites is not cross-linked to another polymer chain. The portion of the polymer chain that is not cross-linked is able to absorb shock and impact, making vinyl esters tougher and more resilient than polyesters.
Having only two ester groups per molecule imparts an additional advantage to vinyl esters. In aqueous environments, ester groups are susceptible to hydrolysis, which causes degradation of the polymer; thus, vinyl esters exhibit better resistance to water and many other chemicals than their polyester counterparts. For these reasons, vinyl esters are often used in highly corrosive environments where other resins fail. Vinyl esters will also function at higher temperatures than polyesters and epoxies. The structure of the polymer between the reactive sites has a profound effect upon the chemical and physical properties of the vinyl ester resin.
The vinyl ester resins most often used in chemical-resistant coatings have an epoxy backbone to which terminal ester end groups are attached. These resins are the reaction products of an addition reaction of an epoxy resin with an unsaturated carboxylic acid, which results in terminal double bonds. Several epoxy resins are used in commerce. The bisphenol a diglycidyl ether epoxy, typically called bis A epoxy, and the epoxylated phenol-formaldehyde novolac, typically called novolac epoxy, are the two most commonly used. The epoxy vinyl ester resins produced are frequently referred to as bis A epoxy vinyl ester resins and novolac epoxy vinyl ester resins respectively. Commonly used acids include acrylic acid, methacrylic acid, isophthalic acid, terephthalic acid, maleic anhydride, and fumaric acid. The physical and chemical properties of the resulting vinyl ester resin depend on the type of epoxy resin used, its molecular weight, and the acid used (Fig. 2).
Fig. 2: The physical and chemical properties of epoxy vinyl ester resins depend on the type of epoxy resin used, its molecular weight, and the acid used.

Other modifiers can be used to impart special properties. For example, toughened vinyl esters can be made by incorporating modified liquid rubbers like carboxy-terminated butadiene-acrylonitrile co-polymers (CTBN), epoxy-terminated butadiene-acrylonitrile rubber (ETBN), core shell rubbers, and certain vinyl-modified hybrid urethanes. Toughening vinyl ester resins is usually done to increase properties like temperature resistance, glass transition temperatures (Tg), heat distortion limits (HDT), water resistance, and fracture toughness. Generally, when additives are used to improve elongation and flexibility, chemical resistance will decrease. Flexibilized polyester and vinyl ester resins are typically limited to formulating primers and are rarely used as topcoats.
There is considerable interest in two newer technologies for curing vinyl ester resins. Styrene is a prohibited ingredient in some applications, such as in potable water or in contact with food, and for some customers due to health considerations.
Cobalt carboxylates have already been classified as “CMR2 Reprotoxic” by the European Chemical Agency (ECHA), which means they are carcinogenic, mutagenic reproductive toxins. In Europe, manufacturers must conform to a wide-ranging directive known as REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals). European manufacturers are under pressure to eliminate cobalt (Co+2) from coatings and composites. All other cobalt compounds will eventually be evaluated, and those that are capable of forming the Co+2 ion will be prohibited. Cobalt napthanate and cobalt octoate will both form the Co+2 ion. This means that manufacturers must search for replacements for both cobalt salts and styrene. Other transition group metals such as copper, manganese, and iron have the ability to start the redox reaction needed to initiate the cure, without having carcinogenic or mutagenic characteristics. Other monomers may be used in place of styrene, some of which are neither hazardous air pollutants (HAPs) nor volatile organic compounds (VOCs), resulting in a “styrene-free” vinyl ester. These other monomers include tert-butyl styrene, vinyl toluene, diallyl phthalate, and trimethylolpropane triacrylate. The latter two are neither HAPs nor VOCs and are more expensive.
The use of ultraviolet light (UV) to cure vinyl ester coatings is gaining in interest and in importance. These coatings also use free radical initiators to initiate cure, but instead of MEKP, a photo initiator is added to the resin. When exposed to UV light, the photo initiators become “excited” and then decompose to generate the free radicals. Radiation-cured resins offer the potential to reduce air pollution and to reduce carcinogens in the environment because they are solvent free. In practice, UV-curable vinyl esters are somewhat limited in their applications because the uncured coating must be exposed to the proper wavelength of UV light at the required intensity. This generally requires placing the UV light source close to the freshly applied coating, while maintaining a uniform distance from the coating. This is not always possible, especially at construction sites.
Various types of fillers are typically added to polyester and vinyl ester coatings to impart distinct characteristics to the coatings and change specific properties of the coating, such as cost, permeation, abrasion resistance, and flexibility (Table 1).
Common Fillers and Properties Imparted[ 此帖被小和尚在2013-12-29 10:38重新编辑 ]