搜到一篇文章,转给大家,关于电化学腐蚀理论的。
Electrochemical corrosion involves two half-cell reactions; an oxidation reaction at the anode and a reduction reaction at the cathode. For iron corroding in water with a near neutral pH, these half cell reactions can be represented as:
Anode reaction: 2Fe => 2Fe2+ + 4e-
Cathode reaction: O2 + 2H2O + 4e- => 4OH-
There are obviously different anodic and cathodic reactions for different alloys exposed to various environments. These half cell reactions are thought to occur (at least initially) at microscopic anodes and cathodes covering a corroding surface. Macroscopic anodes and cathodes can develop as corrosion damage progresses with time.
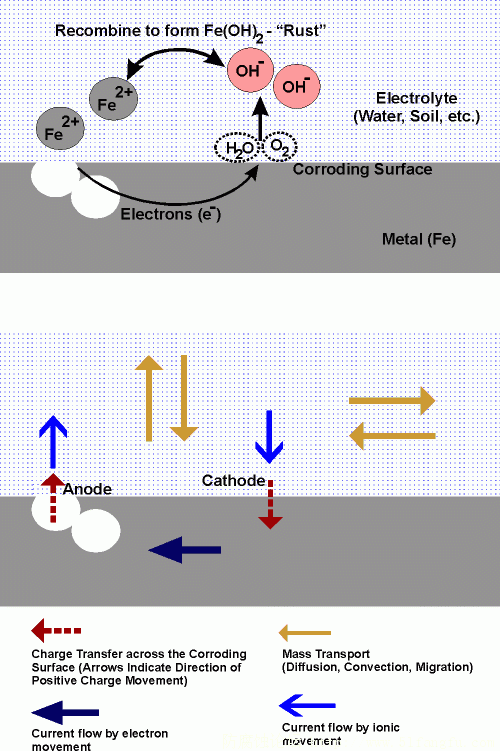
Schematic representation of electrochemical corrosion process
(aqueous corrosion of iron under near neutral pH conditions)
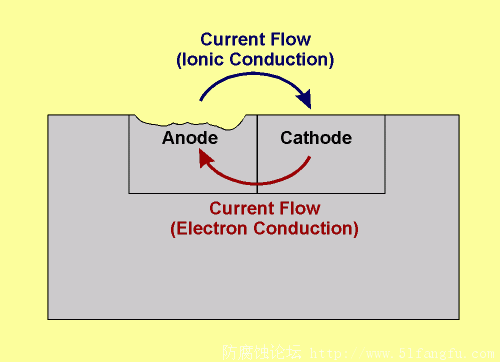
Schematic representation of current flow (conventional current direction)
in a simple corrosion cell
From the above theory it should be apparent that there are four fundamental components in an electrochemical corrosion cell:
An anode.
A cathode.
A conducting environment for ionic movement (electrolyte).
An electrical connection between the anode and cathode for the flow of electron current.
转自:
http://www.corrosion-club.com/basictheory.htm